InVivoMAb anti-mouse IL-18
Product Details
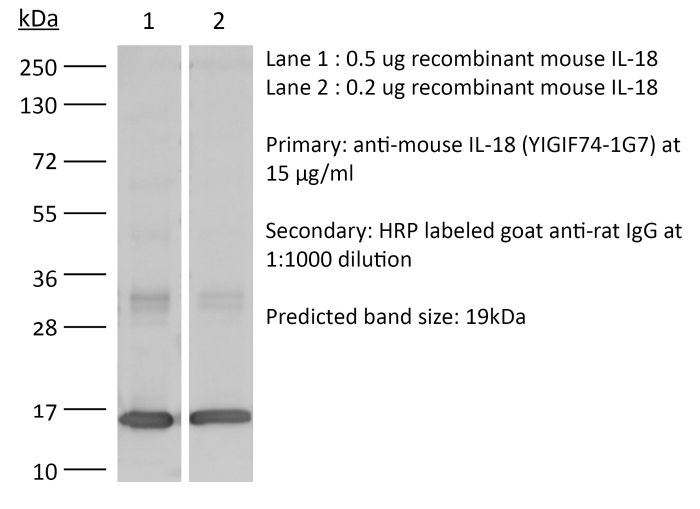
The YIGIF74-1G7 monoclonal antibody reacts with mouse IL-18, an 18 kDa pro-inflammatory cytokine. IL-18 is expressed by activated macrophages, keratinocytes, Kupffer cells, intestinal epithelial cells, and osteoblasts. IL-18 has been shown to activate NF-κB, induce Fas ligand expression, induce both CC and CXC chemokine expression, and enhance the production of IFNγ and GM-CSF.Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vivo IL-18 neutralization in vitro IL-18 neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687719 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IL-18 neutralization
Cohen, T. S., et al. (2018). "S. aureus Evades Macrophage Killing through NLRP3-Dependent Effects on Mitochondrial Trafficking" Cell Rep 22(9): 2431-2441. PubMed
Clinical severity of Staphylococcus aureus respiratory infection correlates with alpha toxin (AT) expression. AT activates the NLRP3 inflammasome; deletion of Nlrp3, or AT neutralization, protects mice from lethal S. aureus pneumonia. We tested the hypothesis that this protection is not due to a reduction in inflammasome-dependent cytokines (IL-1beta/IL-18) but increased bactericidal function of macrophages. In vivo, neutralization of AT or NLRP3 improved bacterial clearance and survival, while blocking IL-1beta/IL-18 did not. Primary human monocytes were used in vitro to determine the mechanism through which NLRP3 alters bacterial killing. In cells treated with small interfering RNA (siRNA) targeting NLRP3 or infected with AT-null S. aureus, mitochondria co-localize with bacterial-containing phagosomes. Mitochondrial engagement activates caspase-1, a process dependent on complex II of the electron transport chain, near the phagosome, promoting its acidification. These data demonstrate a mechanism utilized by S. aureus to sequester itself from antimicrobial processes within the cell.
in vivo IL-18 neutralization
Robinson, K. M., et al. (2018). "The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice" JCI Insight 3(7). PubMed
Secondary bacterial respiratory infections are commonly associated with both acute and chronic lung injury. Influenza complicated by bacterial pneumonia is an effective model to study host defense during pulmonary superinfection due to its clinical relevance. Multiprotein inflammasomes are responsible for IL-1β production in response to infection and drive tissue inflammation. In this study, we examined the role of the inflammasome during viral/bacterial superinfection. We demonstrate that ASC-/- mice are protected from bacterial superinfection and produce sufficient quantities of IL-1β through an apoptosis-associated speck-like protein containing CARD (ASC) inflammasome-independent mechanism. Despite the production of IL-1β by ASC-/- mice in response to bacterial superinfection, these mice display decreased lung inflammation. A neutrophil elastase inhibitor blocked ASC inflammasome-independent production of IL-1β and the IL-1 receptor antagonist, anakinra, confirmed that IL-1 remains crucial to the clearance of bacteria during superinfection. Delayed inhibition of NLRP3 during influenza infection by MCC950 decreases bacterial burden during superinfection and leads to decreased inflammatory cytokine production. Collectively, our results demonstrate that ASC augments the clearance of bacteria, but can also contribute to inflammation and mortality. ASC should be considered as a therapeutic target to decrease morbidity and mortality during bacterial superinfection.
in vivo IL-18 neutralization, in vitro IL-18 neutralization
Molgora, M., et al. (2017). "IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity" Nature 551(7678): 110-114. PubMed
Interleukin-1 receptor 8 (IL-1R8, also known as single immunoglobulin IL-1R-related receptor, SIGIRR, or TIR8) is a member of the IL-1 receptor (ILR) family with distinct structural and functional characteristics, acting as a negative regulator of ILR and Toll-like receptor (TLR) downstream signalling pathways and inflammation. Natural killer (NK) cells are innate lymphoid cells which mediate resistance against pathogens and contribute to the activation and orientation of adaptive immune responses. NK cells mediate resistance against haematopoietic neoplasms but are generally considered to play a minor role in solid tumour carcinogenesis. Here we report that IL-1R8 serves as a checkpoint for NK cell maturation and effector function. Its genetic blockade unleashes NK-cell-mediated resistance to hepatic carcinogenesis, haematogenous liver and lung metastasis, and cytomegalovirus infection.
in vivo IL-18 neutralization
Chudnovskiy, A., et al. (2016). "Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome" Cell 167(2): 444-456 e414. PubMed
While conventional pathogenic protists have been extensively studied, there is an underappreciated constitutive protist microbiota that is an integral part of the vertebrate microbiome. The impact of these species on the host and their potential contributions to mucosal immune homeostasis remain poorly studied. Here, we show that the protozoan Tritrichomonas musculis activates the host epithelial inflammasome to induce IL-18 release. Epithelial-derived IL-18 promotes dendritic cell-driven Th1 and Th17 immunity and confers dramatic protection from mucosal bacterial infections. Along with its role as a “protistic” antibiotic, colonization with T. musculis exacerbates the development of T-cell-driven colitis and sporadic colorectal tumors. Our findings demonstrate a novel mutualistic host-protozoan interaction that increases mucosal host defenses at the cost of an increased risk of inflammatory disease.
- Mus musculus (House mouse),
A pairwise cytokine code explains the organism-wide response to sepsis.
In Nature Immunology on 1 February 2024 by Takahama, M., Patil, A., et al.
PubMed
Sepsis is a systemic response to infection with life-threatening consequences. Our understanding of the molecular and cellular impact of sepsis across organs remains rudimentary. Here, we characterize the pathogenesis of sepsis by measuring dynamic changes in gene expression across organs. To pinpoint molecules controlling organ states in sepsis, we compare the effects of sepsis on organ gene expression to those of 6 singles and 15 pairs of recombinant cytokines. Strikingly, we find that the pairwise effects of tumor necrosis factor plus interleukin (IL)-18, interferon-gamma or IL-1β suffice to mirror the impact of sepsis across tissues. Mechanistically, we map the cellular effects of sepsis and cytokines by computing changes in the abundance of 195 cell types across 9 organs, which we validate by whole-mouse spatial profiling. Our work decodes the cytokine cacophony in sepsis into a pairwise cytokine message capturing the gene, cell and tissue responses of the host to the disease. © 2024. The Author(s).
- Immunology and Microbiology
Fusobacterium nucleatum infection activates the noncanonical inflammasome and exacerbates inflammatory response in DSS-induced colitis.
In European Journal of Immunology on 1 November 2023 by Boonyaleka, K., Okano, T., et al.
PubMed
Caspase activation results in pyroptosis, an inflammatory cell death that contributes to several inflammatory diseases by releasing inflammatory cytokines and cellular contents. Fusobacterium nucleatum is a periodontal pathogen frequently detected in human cancer and inflammatory bowel diseases. Studies have reported that F. nucleatum infection leads to NLRP3 activation and pyroptosis, but the precise activation process and disease association remain poorly understood. This study demonstrated that F. nucleatum infection exacerbates acute colitis in mice and activates pyroptosis through caspase-11-mediated gasdermin D cleavage in macrophages. Furthermore, F. nucleatum infection in colitis mice induces the enhancement of IL-1⍺ secretion from the colon, affecting weight loss and severe disease activities. Neutralization of IL-1⍺ protects F. nucleatum infected mice from severe colitis. Therefore, F. nucleatum infection facilitates inflammation in acute colitis with IL-1⍺ from colon tissue by activating noncanonical inflammasome through gasdermin D cleavage. © 2023 Wiley-VCH GmbH.
- Immunology and Microbiology
A cytotoxic T cell inspired oncolytic nanosystem promotes lytic cell death by lipid peroxidation and elicits antitumor immune responses.
In Nature Communications on 6 September 2023 by Zuo, Z., Yin, H., et al.
PubMed
Lytic cell death triggers an antitumour immune response. However, cancer cells evade lytic cell death by several mechanisms. Moreover, a prolonged and uncontrolled immune response conversely leads to T-cell exhaustion. Therefore, an oncolytic system capable of eliciting an immune response by killing cancer cells in a controlled manner is needed. Here, we establish a micro-scale cytotoxic T-cell-inspired oncolytic system (TIOs) to precisely lyse cancer cells by NIR-light-controlled lipid peroxidation. Our TIOs present antigen-based cell recognition, tumour-targeting and catalytic cell-lysis ability; thus, the TIOs induce oncolysis in vivo. We apply TIOs to preclinical cancer models, showing anti-tumor activity with negligible side-effects. Tumour regression is correlated with a T-cell based anti-tumour immune response and TIOs also improve responses to anti-PD-1 therapy or STING activation. Our study provides insights to design oncolytic systems for antitumour immunity. Moreover, activation of STING can reverse T-cell exhaustion in oncolysis. © 2023. Springer Nature Limited.
- Immunology and Microbiology
Organism-Wide Analysis of Sepsis Reveals Mechanisms of Systemic Inflammation
Preprint on BioRxiv : the Preprint Server for Biology on 2 February 2023 by Takahama, M., Patil, A., et al.
PubMed
SUMMARY Sepsis is a systemic response to infection with life-threatening consequences. Our understanding of the impact of sepsis across organs of the body is rudimentary. Here, using mouse models of sepsis, we generate a dynamic, organism-wide map of the pathogenesis of the disease, revealing the spatiotemporal patterns of the effects of sepsis across tissues. These data revealed two interorgan mechanisms key in sepsis. First, we discover a simplifying principle in the systemic behavior of the cytokine network during sepsis, whereby a hierarchical cytokine circuit arising from the pairwise effects of TNF plus IL-18, IFN-γ, or IL-1β explains half of all the cellular effects of sepsis on 195 cell types across 9 organs. Second, we find that the secreted phospholipase PLA2G5 mediates hemolysis in blood, contributing to organ failure during sepsis. These results provide fundamental insights to help build a unifying mechanistic framework for the pathophysiological effects of sepsis on the body.
- WB,
- Mus musculus (House mouse),
- Immunology and Microbiology
Alveolar macrophages instruct CD8+ T cell expansion by antigen cross-presentation in lung.
In Cell Reports on 13 December 2022 by Kawasaki, T., Ikegawa, M., et al.
PubMed
Lung CD8+ memory T cells play central roles in protective immunity to respiratory viruses, such as influenza A virus (IAV). Here, we find that alveolar macrophages (AMs) function as antigen-presenting cells that support the expansion of lung CD8+ memory T cells. Intranasal antigen administration to mice subcutaneously immunized with antigen results in a rapid expansion of antigen-specific CD8+ T cells in the lung, which is dependent on antigen cross-presentation by AMs. AMs highly express interleukin-18 (IL-18), which mediates subsequent formation of CD103+CD8+ resident memory T (TRM) cells in the lung. In a mouse model of IAV infection, AMs are required for expansion of virus-specific CD8+ T cells and CD103+CD8+ TRM cells and inhibiting virus replication in the lungs during secondary infection. These results suggest that AMs instruct a rapid expansion of antigen-specific CD8+ T cells in lung, which protect the host from respiratory virus infection.Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse)
Integrated proteomic and transcriptomic landscape of macrophages in mouse tissues.
In Nature Communications on 30 November 2022 by Qie, J., Liu, Y., et al.
PubMed
Macrophages are involved in tissue homeostasis and are critical for innate immune responses, yet distinct macrophage populations in different tissues exhibit diverse gene expression patterns and biological processes. While tissue-specific macrophage epigenomic and transcriptomic profiles have been reported, proteomes of different macrophage populations remain poorly characterized. Here we use mass spectrometry and bulk RNA sequencing to assess the proteomic and transcriptomic patterns, respectively, of 10 primary macrophage populations from seven mouse tissues, bone marrow-derived macrophages and the cell line RAW264.7. The results show distinct proteomic landscape and protein copy numbers between tissue-resident and recruited macrophages. Construction of a hierarchical regulatory network finds cell-type-specific transcription factors of macrophages serving as hubs for denoting tissue and functional identity of individual macrophage subsets. Finally, Il18 is validated to be essential in distinguishing molecular signatures and cellular function features between tissue-resident and recruited macrophages in the lung and liver. In summary, these deposited datasets and our open proteome server ( http://macrophage.mouseprotein.cn ) integrating all information will provide a valuable resource for future functional and mechanistic studies of mouse macrophages. © 2022. The Author(s).
- Immunology and Microbiology
Excessive IL-10 and IL-18 trigger hemophagocytic lymphohistiocytosis-like hyperinflammation and enhanced myelopoiesis.
In The Journal of Allergy and Clinical Immunology on 1 November 2022 by Tang, Y., Xu, Q., et al.
PubMed
Hyperinflammation is a life-threatening condition associated with various clinical disorders characterized by excessive immune activation and tissue damage. Multiple cytokines promote the development of hyperinflammation; however, the contribution of IL-10 remains unclear despite emerging speculations for a pathological role. Clinical observations from hemophagocytic lymphohistiocytosis (HLH), a prototypical hyperinflammatory disease, suggest that IL-18 and IL-10 may collectively promote the onset of a hyperinflammatory state. We aimed to investigate the collaborative roles of IL-10 and IL-18 in hyperinflammation. A comprehensive plasma cytokine profile for 87 secondary HLH patients was first depicted and analyzed. We then investigated the systemic and cellular effects of coelevated IL-10 and IL-18 in a transgenic mouse model and cultured macrophages. Single-cell RNA sequencing was performed on the monocytes/macrophages isolated from secondary HLH patients to explore the clinical relevance of IL-10/IL-18-mediated cellular signatures. The therapeutic efficacy of IL-10 blockade was tested in HLH mouse models. Excessive circulating IL-10 and IL-18 triggered a lethal hyperinflammatory disease recapitulating HLH-like phenotypes in mice, driving peripheral lymphopenia and a striking shift toward enhanced myelopoiesis in the bone marrow. IL-10 and IL-18 polarized cultured macrophages to a distinct proinflammatory state with pronounced expression of myeloid cell-recruiting chemokines. Transcriptional characterization suggested the IL-10/IL-18-mediated cellular features were clinically relevant with HLH, showing enhanced granzyme expression and proteasome activation in macrophages. IL-10 blockade protected against the lethal disease in HLH mouse models. Coelevated IL-10 and IL-18 are sufficient to drive HLH-like hyperinflammatory syndrome, and blocking IL-10 is protective in HLH models. Copyright © 2022. Published by Elsevier Inc.
- Immunology and Microbiology,
- Mus musculus (House mouse)
Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection.
In Immunity on 12 July 2022 by Parsa, R., London, M., et al.
PubMed
The intestinal epithelium comprises the body's largest surface exposed to viruses. Additionally, the gut epithelium hosts a large population of intraepithelial T lymphocytes, or IELs, although their role in resistance against viral infections remains elusive. By fate-mapping T cells recruited to the murine intestine, we observed an accumulation of newly recruited CD4+ T cells after infection with murine norovirus CR6 and adenovirus type-2 (AdV), but not reovirus. CR6- and AdV-recruited intraepithelial CD4+ T cells co-expressed Ly6A and chemokine receptor CCR9, exhibited T helper 1 and cytotoxic profiles, and conferred protection against AdV in vivo and in an organoid model in an IFN-γ-dependent manner. Ablation of the T cell receptor (TCR) or the transcription factor ThPOK in CD4+ T cells prior to AdV infection prevented viral control, while TCR ablation during infection did not impact viral clearance. These results uncover a protective role for intraepithelial Ly6A+CCR9+CD4+ T cells against enteric adenovirus. Copyright © 2022 Elsevier Inc. All rights reserved.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Enterocyte-innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium.
In Mucosal Immunology on 1 February 2022 by Gullicksrud, J. A., Sateriale, A., et al.
PubMed
The intestinal parasite, Cryptosporidium, is a major contributor to global child mortality and causes opportunistic infection in immune deficient individuals. Innate resistance to Cryptosporidium, which specifically invades enterocytes, is dependent on the production of IFN-γ, yet whether enterocytes contribute to parasite control is poorly understood. In this study, utilizing a mouse-adapted strain of C. parvum, we show that epithelial-derived IL-18 synergized with IL-12 to stimulate innate lymphoid cell (ILC) production of IFN-γ required for early parasite control. The loss of IFN-γ-mediated STAT1 signaling in enterocytes, but not dendritic cells or macrophages, antagonized early parasite control. Transcriptional profiling of enterocytes from infected mice identified an IFN-γ signature and enrichment of the anti-microbial effectors IDO, GBP, and IRG. Deletion experiments identified a role for Irgm1/m3 in parasite control. Thus, enterocytes promote ILC production of IFN-γ that acts on enterocytes to restrict the growth of Cryptosporidium. © 2021. The Author(s), under exclusive licence to Society for Mucosal Immunology.
- ELISA,
- Mus musculus (House mouse),
- Immunology and Microbiology
Inflammasome activation leads to cDC1-independent cross-priming of CD8 T cells by epithelial cell-derived antigen.
In eLife on 23 December 2021 by Deets, K. A., Nichols Doyle, R., et al.
PubMed
The innate immune system detects pathogens and initiates adaptive immune responses. Inflammasomes are central components of the innate immune system, but whether inflammasomes provide sufficient signals to activate adaptive immunity is unclear. In intestinal epithelial cells (IECs), inflammasomes activate a lytic form of cell death called pyroptosis, leading to epithelial cell expulsion and the release of cytokines. Here, we employed a genetic system to show that simultaneous antigen expression and inflammasome activation specifically in IECs is sufficient to activate CD8+ T cells. By genetic elimination of direct T cell priming by IECs, we found that IEC-derived antigens were cross-presented to CD8+ T cells. However, cross-presentation of IEC-derived antigen to CD8+ T cells only partially depended on IEC pyroptosis. In the absence of inflammasome activation, cross-priming of CD8+ T cells required Batf3+ dendritic cells (conventional type one dendritic cells [cDC1]), whereas cross-priming in the presence of inflammasome activation required a Zbtb46+ but Batf3-independent cDC population. These data suggest the existence of parallel inflammasome-dependent and inflammasome-independent pathways for cross-presentation of IEC-derived antigens. © 2021, Deets et al.
Neutrophil-specific gain-of-function mutations in Nlrp3 promote development of cryopyrin-associated periodic syndrome.
In The Journal of Experimental Medicine on 4 October 2021 by Stackowicz, J., Gaudenzio, N., et al.
PubMed
Gain-of-function mutations in NLRP3 are responsible for a spectrum of autoinflammatory diseases collectively referred to as "cryopyrin-associated periodic syndromes" (CAPS). Treatment of CAPS patients with IL-1-targeted therapies is effective, confirming a central pathogenic role for IL-1β. However, the specific myeloid cell population(s) exhibiting inflammasome activity and sustained IL-1β production in CAPS remains elusive. Previous reports suggested an important role for mast cells (MCs) in this process. Here, we report that, in mice, gain-of-function mutations in Nlrp3 restricted to neutrophils, and to a lesser extent macrophages/dendritic cells, but not MCs, are sufficient to trigger severe CAPS. Furthermore, in patients with clinically established CAPS, we show that skin-infiltrating neutrophils represent a substantial biological source of IL-1β. Together, our data indicate that neutrophils, rather than MCs, can represent the main cellular drivers of CAPS pathology. © 2021 Stackowicz et al.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Adoptive immunotherapy with transient anti-CD4 treatment enhances anti-tumor response by increasing IL-18Rαhi CD8+ T cells.
In Nature Communications on 7 September 2021 by Kim, S. H., Cho, E., et al.
PubMed
Adoptive T cell therapy (ACT) requires lymphodepletion preconditioning to eliminate immune-suppressive elements and enable efficient engraftment of adoptively transferred tumor-reactive T cells. As anti-CD4 monoclonal antibody depletes CD4+ immune-suppressive cells, the combination of anti-CD4 treatment and ACT has synergistic potential in cancer therapy. Here, we demonstrate a post-ACT conditioning regimen that involves transient anti-CD4 treatment (CD4post). Using murine melanoma, the combined effect of cyclophosphamide preconditioning (CTXpre), CD4post, and ex vivo primed tumor-reactive CD8+ T-cell infusion is presented. CTXpre/CD4post increases tumor suppression and host survival by accelerating the proliferation and differentiation of ex vivo primed CD8+ T cells and endogenous CD8+ T cells. Endogenous CD8+ T cells enhance effector profile and tumor-reactivity, indicating skewing of the TCR repertoire. Notably, enrichment of polyfunctional IL-18Rαhi CD8+ T cell subset is the key event in CTXpre/CD4post-induced tumor suppression. Mechanistically, the anti-tumor effect of IL-18Rαhi subset is mediated by IL-18 signaling and TCR-MHC I interaction. This study highlights the clinical relevance of CD4post in ACT and provides insights regarding the immunological nature of anti-CD4 treatment, which enhances anti-tumor response of CD8+ T cells. © 2021. The Author(s).
- Cardiovascular biology,
- Immunology and Microbiology
Mucosal Vaccination Primes NK Cell-Dependent Development of CD8+ T Cells Against Pulmonary Brucella Infection.
In Frontiers in Immunology on 27 July 2021 by Bhagyaraj, E., Wang, H., et al.
PubMed
Past studies with the live, double-mutant B. abortus (znBAZ) strain resulted in nearly complete protection of mice against pulmonary challenge with wild-type (wt) Brucella via a dominant CD8+ T cell response. To understand the contribution innate immune cells in priming CD8+ T cell responses, mice were nasally dosed with wt B. abortus, smooth vaccine strain 19 (S19), or znBAZ, and examined for innate immune cell activation. Flow cytometric analysis revealed that znBAZ, but not wt B. abortus nor S19 infection, induces up to a 5-fold increase in the frequency of IFN-γ-producing NK cells in mouse lungs. These NK cells express increased CXCR3 and Ki67, indicating their recruitment and proliferation subsequent to znBAZ infection. Their activation status was augmented noted by the increased NKp46 and granzyme B, but decreased NKG2A expression. Further analysis demonstrated that both lung caspase-1+ inflammatory monocytes and monocyte-derived macrophages secrete chemokines and cytokines responsible for NK cell recruitment and activation. Moreover, neutralizing IL-18, an NK cell-activating cytokine, reduced the znBAZ-induced early NK cell response. NK cell depletion also significantly impaired lung dendritic cell (DC) activation and migration to the lower respiratory lymph nodes (LRLNs). Both lung DC activation and migration to LRLNs were significantly impaired in NK cell-depleted or IFN-γ-/- mice, particularly the CD11b+ and monocytic DC subsets. Furthermore, znBAZ vaccination significantly induced CD8+ T cells, and upon in vivo NK cell depletion, CD8+ T cells were reduced 3-fold compared to isotype-treated mice. In summary, these data show that znBAZ induces lung IFN-γ+ NK cells, which plays a critical role in influencing lung DC activation, migration, and promoting protective CD8+ T cell development. Copyright © 2021 Bhagyaraj, Wang, Yang, Hoffman, Akgul, Goodwin and Pascual.
- Immunology and Microbiology
Pyroptosis-dependent and -independent cross-priming of CD8sup>+/sup> T cells by intestinal epithelial cell-derived antigen
Preprint on BioRxiv : the Preprint Server for Biology on 8 July 2021 by Deets, K. A., Nichols, R. D., et al.
PubMed
The innate immune system detects pathogens and initiates adaptive immune responses. Inflammasomes are central components of the innate immune system, but whether inflammasomes provide sufficient signals to activate adaptive immunity is unclear. In intestinal epithelial cells (IECs), inflammasomes activate a lytic form of cell death called pyroptosis, leading to epithelial cell expulsion and the release of cytokines. Here we employed a genetic system to show that simultaneous antigen expression and inflammasome activation specifically in IECs is sufficient to activate CD8 + T cells. By genetic elimination of direct T cell priming by IECs, we found that IEC-derived antigens are cross-presented to CD8 + T cells. However, activation of CD8 + T cells by IEC-derived antigen only partially depended on IEC pyroptosis. In the absence of inflammasome activation, cross-priming of CD8 + T cells required Batf3 + dendritic cells (cDC1), whereas cross-priming in the presence of pyroptosis did not. These data suggest the existence of parallel pyroptosis-dependent and pyroptosis-independent but cDC1-dependent pathways for cross-presentation of IEC-derived antigens.
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Pathology
A sustained type I IFN-neutrophil-IL-18 axis drives pathology during mucosal viral infection.
In eLife on 28 May 2021 by Lebratti, T., Lim, Y. S., et al.
PubMed
Neutrophil responses against pathogens must be balanced between protection and immunopathology. Factors that determine these outcomes are not well-understood. In a mouse model of genital herpes simplex virus-2 (HSV-2) infection, which results in severe genital inflammation, antibody-mediated neutrophil depletion reduced disease. Comparative single-cell RNA-sequencing analysis of vaginal cells against a model of genital HSV-1 infection, which results in mild inflammation, demonstrated sustained expression of interferon-stimulated genes (ISGs) only after HSV-2 infection primarily within the neutrophil population. Both therapeutic blockade of IFNα/β receptor 1 (IFNAR1) and genetic deletion of IFNAR1 in neutrophils concomitantly decreased HSV-2 genital disease severity and vaginal IL-18 levels. Therapeutic neutralization of IL-18 also diminished genital inflammation, indicating an important role for this cytokine in promoting neutrophil-dependent immunopathology. Our study reveals that sustained type I interferon (IFN) signaling is a driver of pathogenic neutrophil responses and identifies IL-18 as a novel component of disease during genital HSV-2 infection. © 2021, Lebratti et al.
- Mus musculus (House mouse)
Crosstalk between enterocytes and innate lymphoid cell drives early IFN-γ-mediated control of i>Cryptosporidium/i>
Preprint on BioRxiv : the Preprint Server for Biology on 14 March 2021 by Gullicksrud, J., Sateriale, A., et al.
PubMed
h4>SUMMARY/h4> The intestinal parasite, Cryptosporidium , is a major contributor to global child mortality and causes opportunistic infection in immune deficient individuals. Innate resistance to Cryptosporidium , which specifically invades enterocytes, is dependent on the production of IFN-γ, yet whether enterocytes contribute to parasite control is poorly understood. In this study, utilizing the natural mouse pathogen, Cryptosporidium tyzzeri , we show that epithelial-derived IL-18 synergized with IL-12 to stimulate innate lymphoid cell (ILC) production of IFN-γ. This innate IFN-γ was required for early parasite control. Loss of STAT1 in enterocytes, but not dendritic cells or macrophages, antagonized early parasite control. Transcriptional profiling of enterocytes from infected mice identified an IFN-γ signature and enrichment of anti-microbial effectors like IDO, GBP and IRG. Deletion experiments identified a role for Irgm1/m3 in parasite control. Thus, enterocytes promote ILC production of IFN-γ that acts on enterocytes to restrict the growth of C. tyzzeri .
- Mus musculus (House mouse),
- Cell Biology,
- Immunology and Microbiology
Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation.
In Autophagy on 1 February 2021 by Wang, Z., Zhou, H., et al.
PubMed
The precise mechanism through which macroautophagy/autophagy affects psoriasis is poorly understood. Here, we found that keratinocyte (KC) autophagy, which was positively correlated with psoriatic severity in patients and mouse models and could be inhibited by mitogen-activated protein kinase (MAPK) family inactivation. The impairment of autophagic flux alleviated psoriasisform inflammation. We also found that an autophagy-based unconventional secretory pathway (autosecretion) dependent on ATG5 (autophagy related 5) and GORASP2 (golgi reassembly stacking protein 2) promoted psoriasiform KC inflammation. Moreover, the alarmin HMGB1 (high mobility group box 1) was more effective than other autosecretory proteins in regulating psoriasiform cutaneous inflammation. HMGB1 neutralization in autophagy-efficient KCs eliminated the differences in psoriasiform inflammation between Krt14+/+-Atg5f/f KCs and Krt14Cre/+-atg5f/f KCs, and conversely, recombinant HMGB1 almost completely restored psoriasiform inflammation in Krt14Cre/+-atg5f/f KCs in vivo. These results suggest that HMGB1-associated autosecretion plays a pivotal role in cutaneous inflammation. Finally, we demonstrated that Krt14Cre/+-hmgb1f/f mice displayed attenuated psoriatic inflammation due to the essential crosstalk between KC-specific HMGB1-associated autosecretion and γδT cells. Thus, this study uncovered a novel autophagy mechanism in psoriasis pathogenesis, and the findings imply the clinical significance of investigating and treating psoriasis.Abbreviations: 3-MA: 3-methyladenine; ACTB: actin beta; AGER: advanced glycosylation end-product specific receptor; Anti-HMGB1: anti-HMGB1 neutralizing antibody; Anti-IL18: anti-IL18 neutralizing antibody; Anti-IL1B: anti-IL1B neutralizing antibody; ATG5: autophagy related 5; BAF: bafilomycin A1; BECN1: beclin 1; CASP1: caspase 1; CCL: C-C motif chemokine ligand; CsA: cyclosporine A; ctrl shRNA: lentivirus harboring shRNA against control; CXCL: C-X-C motif chemokine ligand; DCs: dendritic cells; DMEM: dulbecco's modified Eagle's medium; ELISA: enzyme-linked immunosorbent assay; EM: electron microscopy; FBS: fetal bovine serum; GORASP2 shRNA: lentivirus harboring shRNA against GORASP2; GORASP2/GRASP55: golgi reassembly stacking protein 2; GR1: a composite epitope between LY6 (lymphocyte antigen 6 complex) locus C1 and LY6 locus G6D antigens; HE: hematoxylin and eosin; HMGB1: high mobility group box 1; HMGB1 shRNA: lentivirus harboring shRNA against HMGB1; IFNG/IFN-γ: interferon gamma; IL17A: interleukin 17A; IL18: interleukin 18; IL1A/IL-1α: interleukin 1 alpha; IL1B/IL-1β: interleukin 1 beta; IL22/IL-22: interleukin 22; IL23A: interleukin 23 subunit alpha; IL23R: interleukin 23 receptor; IMQ: imiquimod; ITGAM/CD11B: integrin subunit alpha M; ITGAX/CD11C: integrin subunit alpha X; IVL: involucrin; KC: keratinocyte; KD: knockdown; KO: knockout; Krt14+/+-Atg5f/f mice: mice bearing an Atg5 flox allele, in which exon 3 of the Atg5 gene is flanked by two loxP sites; Krt14+/+-Hmgb1f/f: mice bearing an Hmgb1 flox allele, in which exon 2 to 4 of the Hmgb1 gene is flanked by two loxP sites; Krt14Cre/+-atg5f/f mice: keratinocyte-specific atg5 knockout mice generated by mating Atg5-floxed mice with mice expressing Cre recombinase under the control of the promoter of Krt4; Krt14Cre/+-hmgb1f/f mice: keratinocyte-specific hmgb1 knockout mice generated by mating Hmgb1-floxed mice with mice expressing Cre recombinase under the control of the promoter of Krt14; Krt14-Vegfa mice: mice expressing 164-amino acid Vegfa splice variant recombinase under the control of promoter of Krt14; LAMP1: lysosomal associated membrane protein 1; LDH: lactate dehydrogenase; LORICRIN: loricrin cornified envelope precursor protein; M5: TNF, IL1A, IL17A, IL22 and OSM in combination; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MAPK: mitogen-activated protein kinase; MKI67: marker of proliferation Ki-67; MTT: thiazolyl blue tetrazolium bromide; NFKB/NF-κB: nuclear factor kappa B; NHEKs: primary normal human epidermal keratinocytes; NS: not significant; OSM: oncostatin M; PASI: psoriasis area and severity index; PtdIns3K: class III phosphatidylinositol 3-kinase; qRT-PCR: quantitative RT-PCR; RELA/p65: RELA proto-oncogene, NF-kB subunit; rHMGB1: recombinant HMGB1; rIL18: recombinant interleukin 18; rIL1B: recombinant interleukin 1 beta; S100A: S100 calcium binding protein A; SQSTM1/p62: sequestosome 1; T17: IL17A-producing T; TCR: T-cell receptor; tcrd KO mice: tcrd (T cell receptor delta chain) knockout mice, which show deficient receptor expression in all adult lymphoid and epithelial organs; TLR: toll-like receptor; TNF/TNF-α: tumor necrosis factor; WOR: wortmannin; WT: wild-type; γδT17 cells: IL17A-producing γδ T cells.
- Immunology and Microbiology,
- Pathology
A sustained type I IFN-neutrophil-IL-18 axis drives pathology during mucosal viral infection
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2020 by Lebratti, T. J., Lim, Y. S., et al.
PubMed
h4>ABSTRACT/h4> Neutrophil responses against pathogens must be balanced between protection and immunopathology. Factors that determine these outcomes are not well-understood. In a mouse model of genital herpes simplex virus-2 (HSV-2) infection, which results in severe genital inflammation, antibody-mediated neutrophil depletion reduced disease. Comparative single cell RNA-sequencing analysis of vaginal cells against a model of genital HSV-1 infection, which results in mild inflammation, demonstrated sustained expression of interferon-stimulated genes (ISGs) only after HSV-2 infection primarily within the neutrophil population. Both therapeutic blockade of IFN α/β receptor 1 (IFNAR1) and genetic deletion of IFNAR1 in neutrophils concomitantly decreased HSV-2 genital disease severity and vaginal IL-18 levels. Therapeutic neutralization of IL-18 also diminished genital inflammation, indicating an important role for this cytokine in promoting neutrophil-dependent immunopathology. Our study reveals that sustained type I IFN signaling is a driver of pathogenic neutrophil responses, and identifies IL-18 as a novel component of disease during genital HSV-2 infection.
- Mus musculus (House mouse),
- Immunology and Microbiology
Treatment of mice with S4B6 IL-2 complex prevents lethal toxoplasmosis via IL-12- and IL-18-dependent interferon-gamma production by non-CD4 immune cells.
In Scientific Reports on 4 August 2020 by Kupz, A., Pai, S., et al.
PubMed
Toxoplasmic encephalitis is an AIDS-defining condition. The decline of IFN-γ-producing CD4+ T cells in AIDS is a major contributing factor in reactivation of quiescent Toxoplasma gondii to an actively replicating stage of infection. Hence, it is important to characterize CD4-independent mechanisms that constrain acute T. gondii infection. We investigated the in vivo regulation of IFN-γ production by CD8+ T cells, DN T cells and NK cells in response to acute T. gondii infection. Our data show that processing of IFN-γ by these non-CD4 cells is dependent on both IL-12 and IL-18 and the secretion of bioactive IL-18 in response to T. gondii requires the sensing of viable parasites by multiple redundant inflammasome sensors in multiple hematopoietic cell types. Importantly, our results show that expansion of CD8+ T cells, DN T cells and NK cell by S4B6 IL-2 complex pre-treatment increases survival rates of mice infected with T. gondii and this is dependent on IL-12, IL-18 and IFN-γ. Increased survival is accompanied by reduced pathology but is independent of expansion of TReg cells or parasite burden. This provides evidence for a protective role of IL2C-mediated expansion of non-CD4 cells and may represent a promising lead to adjunct therapy for acute toxoplasmosis.
- Biochemistry and Molecular biology,
- Cardiovascular biology
Cardiac β-adrenergic receptor activation mediates distinct and cell type-dependent changes in the expression and distribution of connexin 43.
In Journal of Cellular and Molecular Medicine on 1 August 2020 by Zhang, Y., Hou, M. C., et al.
PubMed
Activation of the sympatho-β-adrenergic receptors (β-ARs) system is a hallmark of heart failure, leading to fibrosis and arrhythmias. Connexin 43 (Cx43) is the most abundant gap junctional protein in the myocardium. Current knowledge is limited regarding Cx43 remodelling in diverse cell types in the diseased myocardium and the underlying mechanism. We studied cell type-dependent changes in Cx43 remodelling due to β-AR overactivation and molecular mechanisms involved. Mouse models of isoproterenol stimulation or transgenic cardiomyocyte overexpression of β2 -AR were used, which exhibited cardiac fibrosis and up-regulated total Cx43 abundance. In both models, whereas Cx43 expression in cardiomyocytes was reduced and more laterally distributed, fibroblasts exhibited elevated Cx43 expression and enhanced gap junction communication. Mechanistically, activation of β2 -AR in fibroblasts in vitro elevated Cx43 expression, which was abolished by the β2 -antagonist ICI-118551 or protein kinase A inhibitor H-89, but simulated by the adenylyl cyclase activator forskolin. Our in vitro and in vivo data showed that β-AR activation-induced production of IL-18 sequentially stimulated Cx43 expression in fibroblasts in a paracrine fashion. In summary, our findings demonstrate a pivotal role of β-AR in mediating distinct and cell type-dependent changes in the expression and distribution of Cx43, leading to pathological gap junction remodelling in the myocardium. © 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley Sons Ltd.